Who we are
Ultupharma AB was founded in 2015 as a collaboration with researchers at the Swedish University of Agricultural Sciences (SLU) with the goal to find new antibiotics in nature. The work is carried out at SLU, by collaborating university groups and by contract laboratories, in several countries.
What we do
Ultupharma has the goal to develop antibiotics with new mechanisms of action using two different approaches:
1) New antibiotics based on compounds produced by microorganisms.
2) New antibiotics by repurposing nucleoside analogues in combination with other known compounds.
The Ultupharma ULT1, ULT2 and ULT3-programmes have several patended antibiotics with new mechanism of action, active against multi-drug-resistant bacteria.
Antibiotic resistance
The biggest threat to modern medicine
There is an urgent global need for new antibiotics. The spread of multiresistant bacteria is threatening the basis of modern healthcare, and the global antibiotic pipeline is simply not keeping up with the increase in resistance.
In September 2016 all 193 United Nation members agreed that antibiotic resistance was the biggest threat to modern medicine. Effective treatment of bacterial infections is the cornerstone of modern medicine, but the spread of multiresistant bacteria has already started to seriously limit the possibilities to treat life-threatening infectious diseases. Antibiotic resistance will also severely decrease the possibilities to perform certain types of surgery, transplant organs or stem cells, use chemotherapy or treatments with biological pharmaceuticals for patients with cancer or autoimmune diseases, etc.
Three years ago it was reported in the Lancet [Murray et al., Lancet 399:629-655 (2022)]: "The six leading pathogens for deaths associated with resistance (Escherichia coli, followed by Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa) were responsible for 929 000 (660 000–1 270 000) deaths attributable to AMR and 3.57 million (2.62–4.78) deaths associated with AMR in 2019. One pathogen, meticillin-resistant S. aureus, caused more than 100 000 deaths attributable to AMR in 2019, while six more each caused 50 000–100 000 deaths: multidrug-resistant excluding extensively drug-resistant tuberculosis, third-generation cephalosporin-resistant E. coli, carbapenem-resistant A. baumannii, fluoroquinolone-resistant E. coli, carbapenem-resistant K. pneumoniae, and third-generation cephalosporin-resistant K. pneumoniae."
A failure to address the problem of antibiotic resistance could result in 10 million deaths annually by 2050, and an estimated cost of 100 trillion USD (about 1% of global GDP).
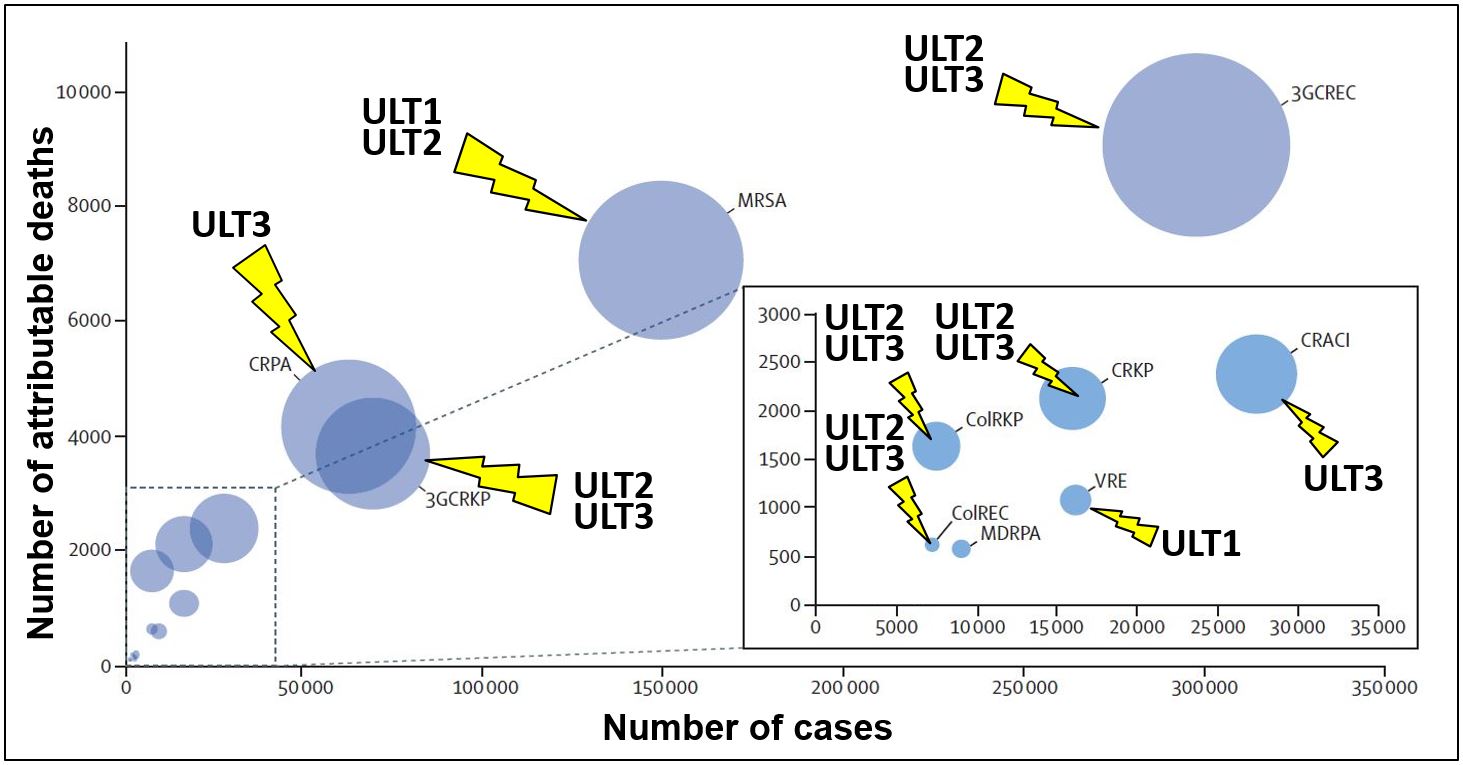
Figure 1 (click image to enlarge). Bubbles indicate the number of cases and deaths attributable to antibiotic-resistant bacteria in EU and the European Economic Area, 2015. (Bubble diameters = disability-adjusted life-years.) Activity of ULT1, ULT2 and ULT3 is indicated. CRACI = carbapenem-resistant Acinetobacter spp. VRE = vancomycin-resistant Enterococcus faecalis and E. faecium. ColREC = colistin-resistant Escherichia coli. 3GCREC = third-generation cephalosporin-resistant E. coli. ColRKP = colistin-resistant Klebsiella pneumoniae. CRKP = carbapenem-resistant K. pneumoniae. 3GCRKP = third-generation cephalosporin-resistant K. pneumoniae. CRPA = carbapenem-resistant P. aeruginosa. MDRPA = multidrug-resistant P. aeruginosa. MRSA = meticillin-resistant Staphylococcus aureus. Adapted from Lancet, Nov. 5, 2018, and with arrows indicating effects of Ultupharma antibiotics.
IDSA (Infectious Diseases Society of America) issued the 10 × ’20 Initiative in 2010, stating that ten new systemic antibiotic drugs were needed by 2020, and then at least one new drug annually.
Despite the growing need for new antibiotics, the development of new antibiotic drugs has been neglected for many years, in spite of multidrug resistant bacteria becoming a rapidly growing problem with an increasingly high cost to society (Figure 2). Since 2010 only three new antibiotics with new mechanisms of action have been approved by FDA (fidaxomicin, 2011; pretomanid and lefamulin, both 2019). Other drugs have either been members of existing classes of antibiotics or old antibiotics combined with new beta lactamase inhibitors. A report from the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) 2024 (Figure 3, below) shows a serious lack of new antibiotics in clinical development.
Several recent financial initiatives and new market possibilities will contribute to making development of new antibiotic drugs important and profitable not only to society but also to pharmaceutical companies.
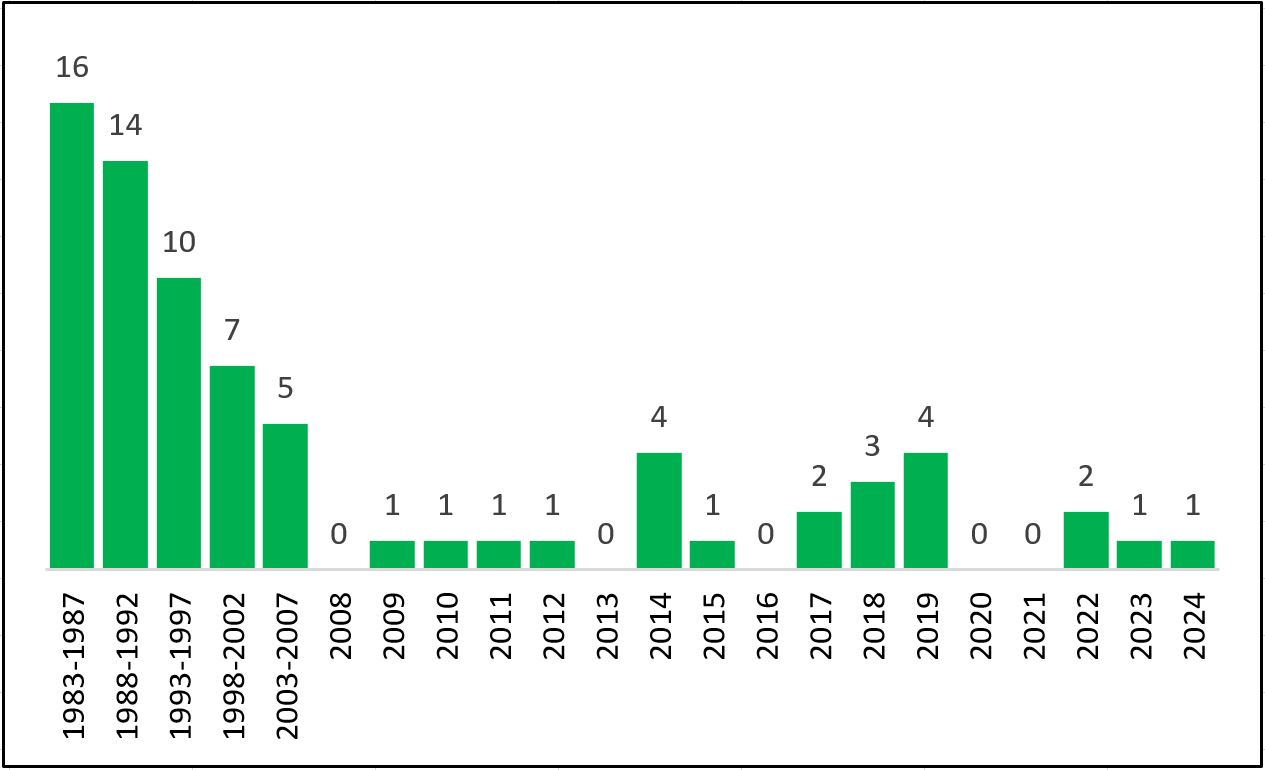
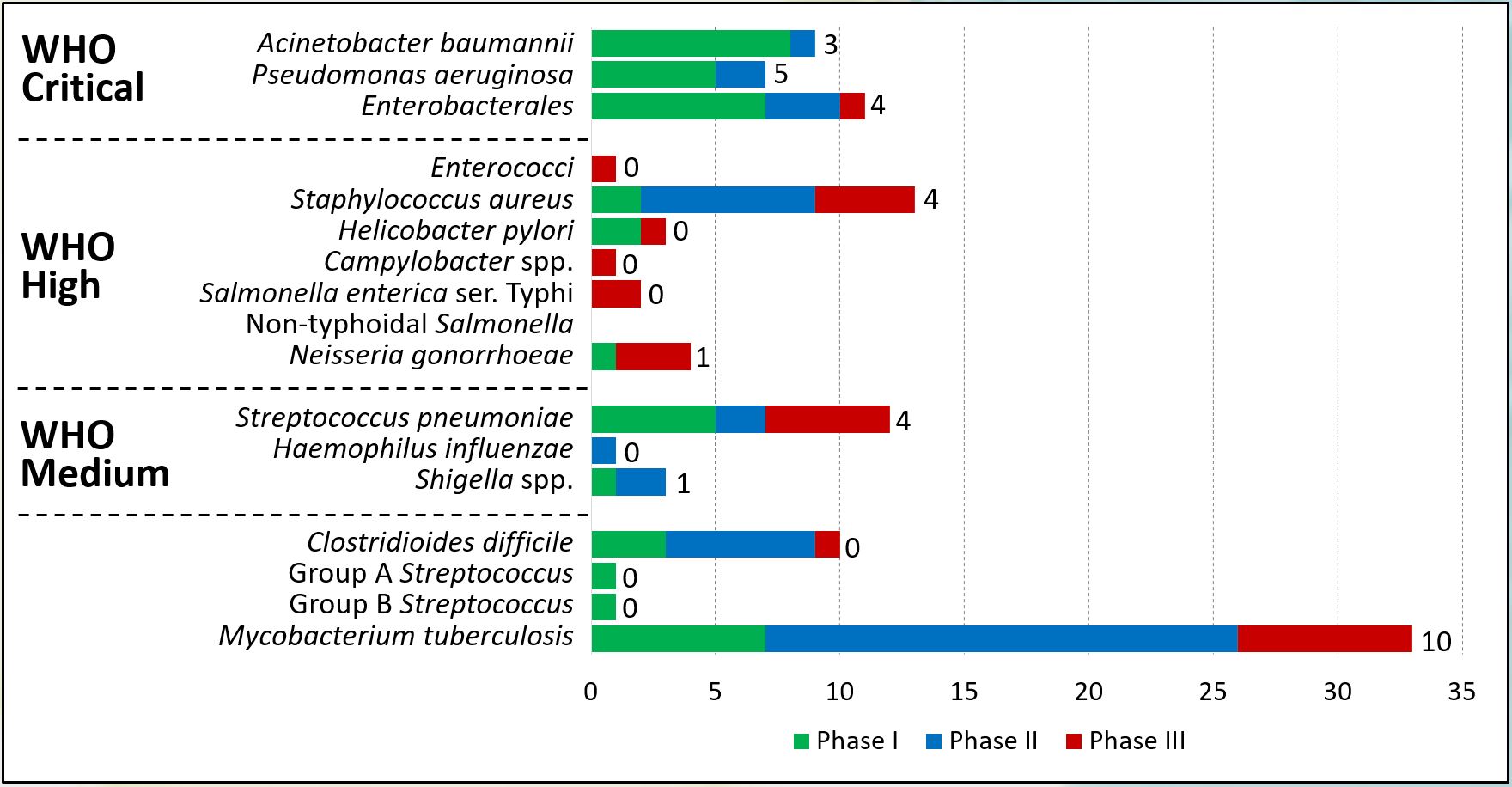
Figure 3 (click image to enlarge). Data from the IFPMA 2024-report (https://www.ifpma.org/wp-content/uploads/2024/06/20240514_From-Resistance-to-Resilience_AMR.pdf), showing the serious lack of new antibiotics in clinical development. Numbers next to bars show the number of WHO innovative candidates, i.e. i) new chemical class, ii) new target, iii) new mode of action, and/or iv) no cross-resistance to other antibiotic classes. These are scaringly low numbers of innovative candidates.
